|
ARISE Fluids Study Australasian Resuscitation in Sepsis Evaluation: Fluids or Vasopressors in Emergency Department Sepsis A randomised controlled trial Recruitment Complete November 2025 |
|
AIMS
- P In patients with septic shock presenting to the emergency department
- I does an early vasopressor/fluid-sparing resuscitation strategy
- C compared to a fluid unrestricted/later vasopressor regimen
- O Improve hospital-free survival and functional outcome?
|
Inclusion Criteria |
Exclusion Criteria |
|
|
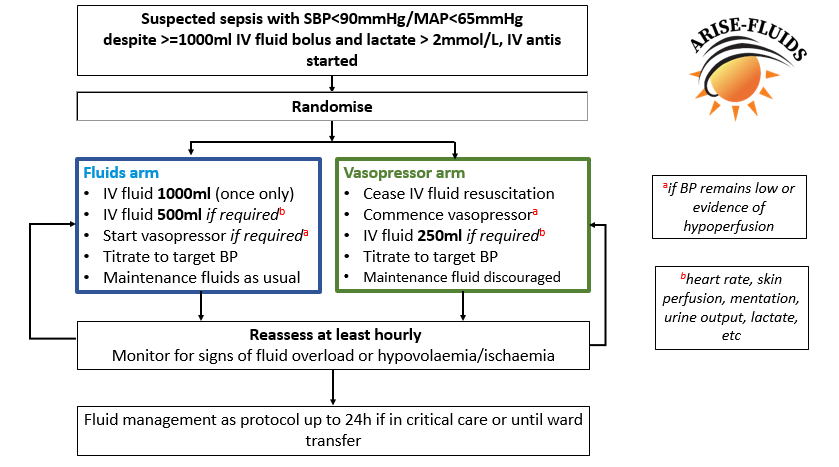
What you need to do:
- Any patient that meets inclusion criteria either:
- Randomise the patient or
- contact the research team on 28608 to assist
- To Randomise a patient:
- Log onto www.arisefluids.org User Name: rdpharise Password: AriseArise
- Randomise the patient
- Print randomisation result
- Grab the relevant study arm pack from research draw in resus (dependent on which arm the patient was randomised to)
- Attach intervention arm laminate to IV pole
- Put handover form in notes
- Text the research team with patients name, HRN and study ID number
- Patient to stay on allocated arm for
- Minimum 6 hours
- Maximum 24 hours if in ED/ICU
Further Questions
- Prinicipal Investigator: Dr Sandra Brownlea
This email address is being protected from spambots. You need JavaScript enabled to view it. -
- Megan Duck; Research Coordinator (
This email address is being protected from spambots. You need JavaScript enabled to view it. ) 28608 - Hanna Bjoern; Research nurse (hanna.bjoern
This email address is being protected from spambots. You need JavaScript enabled to view it. ) 27969 This email address is being protected from spambots. You need JavaScript enabled to view it. 0460034800
- Megan Duck; Research Coordinator (

